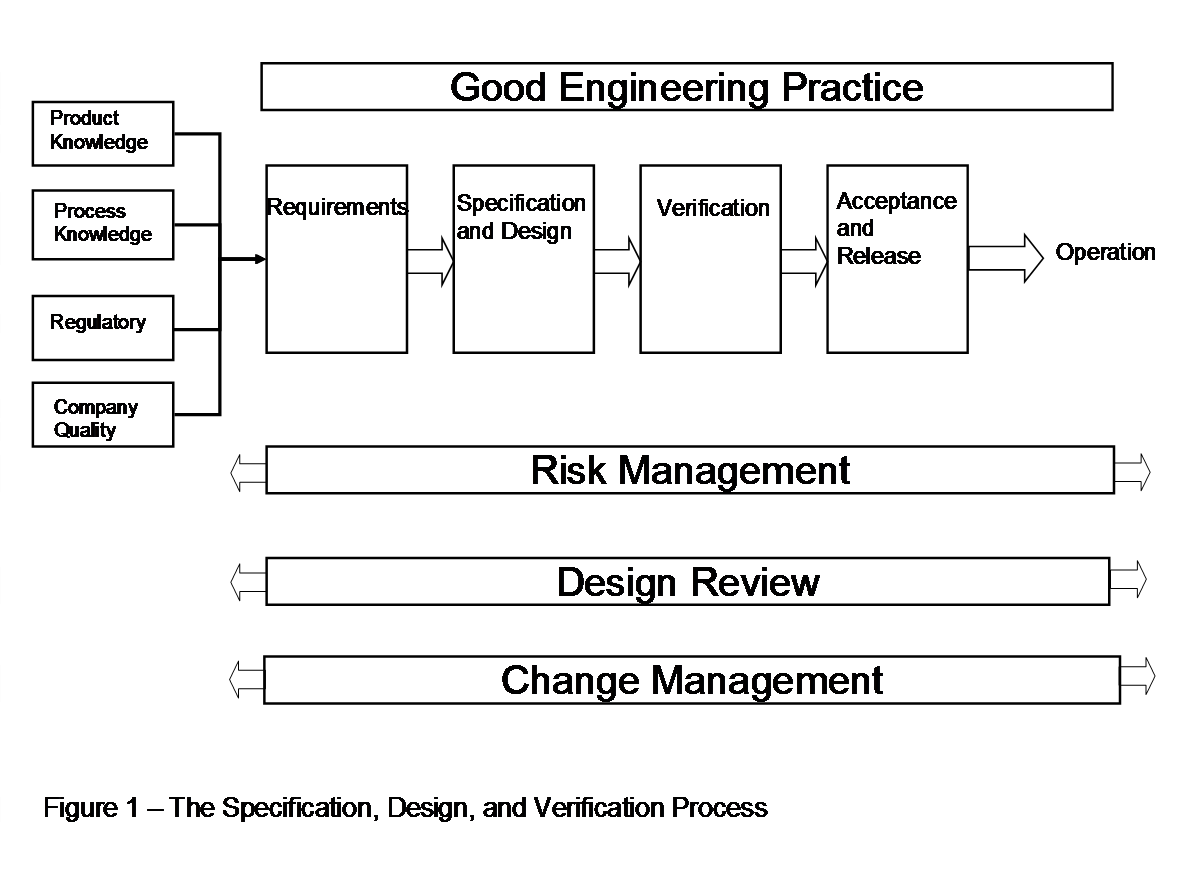
ASTM E2500-7 is a consensus guide with legal reference and global impact. The has Copyright by ASTM Int'l (all rights reserved) and can be purchased at www.astm.org.
The ASTM E2500 has a lean approach for validation and is in line with FDA’s Pharmaceutical cGMP's for the 21st century, EMEA, GAMP 5 and ICH Q8 and Q9.